SOLVED:Consider The Following Reaction For The Co
This chemistry video tutorial explains how to find the amount of excess reactant that is left over after the reaction is complete. You need to start with...uncountable noun octane Octane is a chemical substance that exists in petrol or gasoline and that is used to measure the quality of the fuel. after the reaction how much octane is left?Express your answer with the appropriate units. > View Available Hint(s) H20 produced = 0.547 mol Submit Previous Answers Figure <1 of 1 (> All attempts used; correct answer displayed UNLEADED Part D $ 26.23 9 9.234 2.841 After the reaction, how much octane is left?0.660 mol of octane is allowed to react with .780 mol of oxygen. Oxygen is the limiting reactant. Concepts and reason This is based on the concept that the limiting reagent decides how much amount of other reactants will be consumed when the reaction takes place.Octane number, also called octane value or octane rating, is one of the critical measures of a gasoline's performance. It is a measure of a fuel's resistance to knock or to ignite prematurely.
Octane Definition. The meaning of Octane - Word Panda
The short answer is that octane is the measure of how much compression a fuel can withstand before igniting. Or, in layman's terms, the higher the octane rating, the less likely the fuel is going to pre-ignite (read: explode unexpectedly) at higher pressures and damage your engine. That's why performance...Octane is one of the alkane group of hydrocarbons and is a major component of petrol (gasoline). Burning octane gives this chemical reaction: 2.C8H18 + 25.O2 = 16.CO2 + 18.H2O The formula shows that 1 kg of octane burns with 3.51 kg oxygen to produce 3.09 kg shift the reaction to the left.Octane ratings increase in aromatics with same number of carbons. It is important to know octane numbers for petrol because the auto ignition of fuels causes a �knocking� effect in petrol engines. This is where it ignites twice; once due to the high pressure and again when the spark ignites the petrol..208mol of octane is allowed to react with .780 mol of oxygen. Oxygen is the limiting reactant. Subtract that amount from 0.208 moles of octane, and you get 0.208 - [0.708 x (25/2)] = 0.151 moles of octane left. If you need to give the number of grams, multiply that number by the molecular weight of...

[Solved] The octane rating of gasoline is a relationship... | Course Hero
Now there are only ruins left of Hadrian's Wall. 4. The largest development in the debate among scientists about what killed the prehistoric dinosaurs 1. The new_magazine is becoming more and more popular. week 2. Jeff felt terrible because he was _to help his friend...After the reaction, how much octane is left? Ammonia gas reacts with oxygen gas according to the following equation: 4NH3 + 5O2----4NO + 6H2O a. How many moles of oxygen gas are needed to react with 23 moles of ammonia?Midway through the WWII season, Octane left Luminosity Gaming to join OpTic Gaming, which was the biggest move of his career at the time. However, after a difficult WWII season, Octane was dropped as OpTic decided to revamp their roster for the Black Ops 4 season. Now a member of 100 Thieves'...Science. Chemistry Q&A Library After the reaction, how much octane is left? Here is the balanced equation: 2C8H18(g)+25O2(g)→16CO2(g)+18H2O(g) number of moles of C8H18 reacted number of moles of C8H18 reacted = 6.16×10−2 mol. H2O produced = 0.554 mol. oxygen is the limiting reactant.Q. How many elements make up the continent of Teyvat? 7. Q. Which of the following cannot Q. The Sunsettia is the most commonly found food in the game. It has no other uses apart from being eaten Q. How many balloons can be in a row in balloons crash event of Windblume Festival something.
Chantel,
You are totally correct that oxygen is the limiting reagent: for every 25 moles of oxygen, you use up two moles of octane. That means for 0.780 moles of oxygen, you use up 0.708 x 2/25 moles of oxygen = 0.0566 moles of octane.
Subtract that amount from 0.208 moles of octane, and you get 0.208 - [0.708 x (25/2)] = 0.151 moles of octane left.
If you need to give the number of grams, multiply that number by the molecular weight of octane, 114.23: 114.23 x 0.151 = 17.3 grams.
Hope this helped!
Solved: Balanced Chemical Equation. 2C8H18(g) + 25O2(g ...

Problems in everyday life that need solving - Smart ...

CHM 110 - Chemistry and Issues in the Environment Charles ...

The inexplicable vaccine hesitancy across much of the ...

Solved: 0.130 Mol Of Octane Is Allowed To React With 0.790 ...

0.660 mol of octane is allowed to react with .780 mol of ...

Solved: Phosphorus - 32 Is A Reaction Isotope Used As A Tr ...
Octane VI 17.3 guide(not quite) for better cooling ...
High travel, high clearance & high octane, a streetable ...
Chemistry Archive | July 02, 2014 | Chegg.com

Annie Kennedy talks of life working with Ferrary in F1 ...
Answered: d. How much excess reactant is left… | bartleby

Kim Kardashian rumoured to be dating CNN presenter Van Jones

The Octane Rating Of Gasoline Is A Relationship Of ...
Chemistry: The Central Science, Chapter 1, Section 2

(PDF) Solucionario Quimica General - Rymond Chang 10e ...

Chemistry Archive | July 14, 2017 | Chegg.com

MNL All Stars 2-1 Leeds United player ratings: Low-octane ...

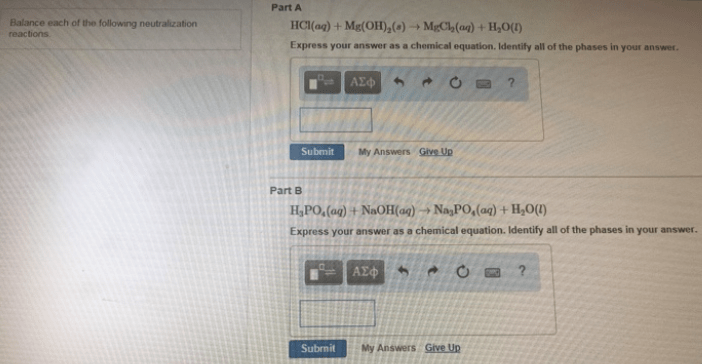
Balance each of the following neutralizati... | Clutch Prep
Chemistry Archive | October 30, 2017 | Chegg.com

Express your answer with the appropriate units ANSWER ...


Aucun commentaire:
Enregistrer un commentaire
Remarque : Seul un membre de ce blog est autorisé à enregistrer un commentaire.