O2 2- Lewis Structure: How To Draw The Lewis Structure For O2 2
+10 pts. Answered. Lewis structure for 2O2 I'm okay with making lewis structures where there will be one 'thing' in the middle but in 2O2 I'm unsure because there are 2 oxygen's in the middle but is there an oxygen on either side or on top of the middle oxygen's? I'm unsure about the the difference...(Redirected from Lewis structures). Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.Lewis Dot structures. One of the early questions asked by scientists, once the concepts of atoms and molecules had been firmly established was "How are Atoms Bonded?" Try using these rules to create Lewis structures for the following: CO32-, O2, AlCl3, IF3, XeF6.The Lewis structure of a compound can be generated by trial and error. We start by writing symbols that contain the correct number of valence electrons for the atoms in the molecule. We then combine electrons to form covalent bonds until we come up with a Lewis structure in which all of the elements...7. In drawing Lewis structures for relatively small molecules and polyatomic ions, the structures tend to be more stable when they are compact and symmetrical rather than extended chains of atoms. EXAMPLE: Write the Lewis structure for CH2O where carbon is the central atom.
Lewis structure - Wikipedia
Lewis dot structure is the classical bonding model in which only valence electrons of the atoms are used. Hence it is very important to learn how to draw Lewis Dot structure correctly for an atom, ion, molecule, polyatomic ion and an ionic compound.A Example of LEWIS DOT STRUCTURES 1. Arrange the symbols such that the least electronegative element is in the center and the other elements are surrounding the central atom. O C O 2. Count the total number of electrons from the valence electrons.Lewis structures show how the electrons are arranged in the molecule. The diagram opposite shows the Lewis structure for the water molecule, H2O Dots represent electrons which are not involved in bonding. Most commonly, two occur in pairs and then represent non-bonding or lone pairs (such as...In nature, the Lewis structure for oxygen exists this way so it could react with other elements and in nature, it is a gas so it exists as O2 . As you can see, O2, each oxygen is connected by a single bond, each gets two octets, and on the bottom of each O, each O has one unpaired electron.

Molecular Structure: Lewis Dot
The structure on the right is the Lewis electron structure , or Lewis structure , for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons.Chemical Bonding: O22- Lewis Structure. With O 2 2- be sure to add two additional valence electrons to your total because of the negative two in the chemical formula.If you want to know methods of determining Lewis structures, I'll be glad to point you in the right direction or do examples with you. of dots under the second O. An interesting feature of the O2 molecule, which cannot be explained from the dot structure, is that oxygen molecules are...These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds. Principal Energy Levels. An atom consists of a positively charged nucleus and negatively charged electrons.Lewis structures are structural formulas for molecules and polyatomic ions that represent all valence electrons. Since valence electrons are typically represented as dots, these structural formulas sometimes are called Lewis dot stutctures. Here we present some examples of how to draw Lewis...
Error
This document was not found on this server.We hope the links on the left and those below will be useful in helping you find the information you are after:
Please contact the webmanager if you require further help or to report a problem such as a dead link.What Is The Lewis Structure For OCN-? | Socratic

Carbon Monoxide Lewis Structure (Page 1) - Line.17QQ.com

Peroxide Lewis Structure (Page 1) - Line.17QQ.com

Solved: Answer The Next TWO Questions Based On The Most Pl... | Chegg.com

Chemical Bonding Chapter 9
A. Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic. B_2^2+, B2, C_2^2-, B_2^2- And N_2^2+ B. Draw The Lewis Structures And Molecular Orbital Diagrams For |

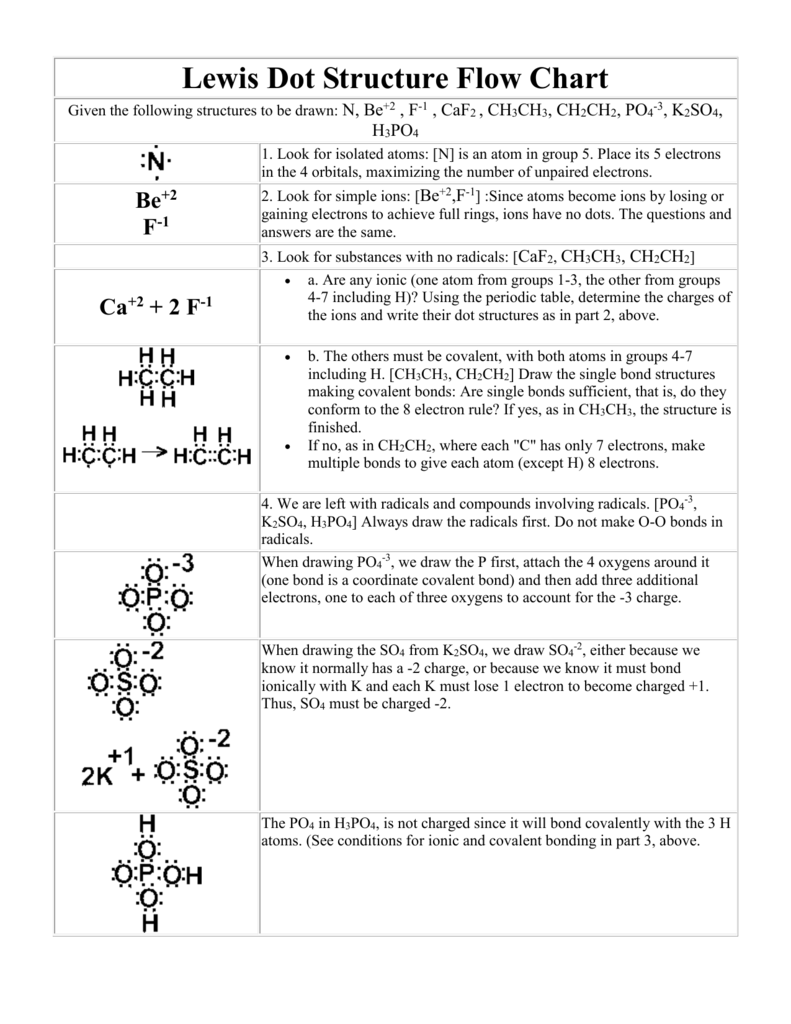
Lewis Dot Structure Flow Chart - Wikispaces
Chapter 9 Homework Chemistry.pdf - Chapter 9 Homework 9.26 Draw The Lewis Structures For Each Of The Following Molecules Or Ions And Predict Their | Course Hero

Lewis Structure For Chloroform (Page 1) - Line.17QQ.com

1 Reactants Reaction Products 2 H2 2 H2O O2 + 2
問答題 1) Write The Lewis Structures (including Lone Pairs

7 - Wiley

Explain The Following.The O22+ Ion Has A S... | Clutch Prep

How To Determine The Lewis Dot Structure Of SO3 - Quora

Solved: I Am Not Understanding What I Am Doing Wrong For O... | Chegg.com
Molecular Orbital Theory -- Homodiatomics Use The Molecular Orbital Model To Fully Describe The Bonding In O2+, O2, O2-, And O22-. Determine Which Of The Following Statements Are True And Which Are

Show The Formation Of The Following Molecules With The Help Of Electron Dot Structure (a) CH4 (b) CO2 - Brainly.in

Short Answer Type Questions – I
Which Of The Following Molecules Or Ions Contain Polar Bonds A O3 No E CO2 Yes | Course Hero

So4 2 Lewis Structure (Page 1) - Line.17QQ.com

Triplet Oxygen - Wikipedia


Aucun commentaire:
Enregistrer un commentaire
Remarque : Seul un membre de ce blog est autorisé à enregistrer un commentaire.