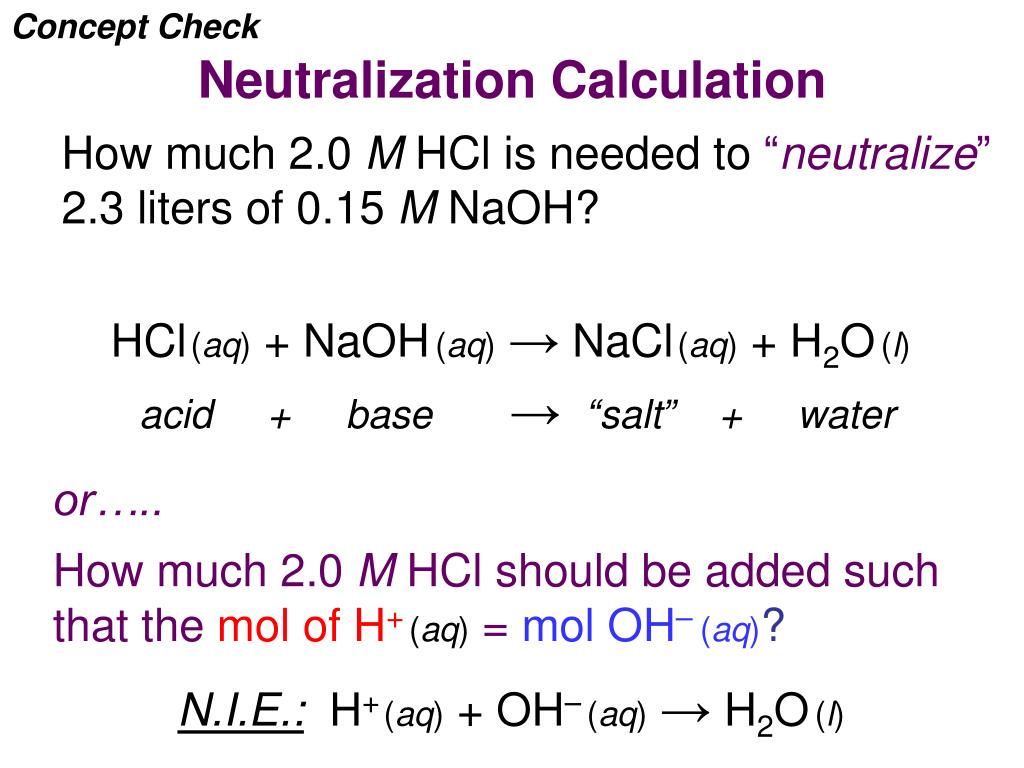
H2SO4(aq) + 2NaOH(aq) Na2SO4(aq)... | Wyzant Ask An Expert
Solution. Because there are two OH − ions in the formula for Ca(OH) 2 , we need two moles of HNO 3 to provide H + ions. 8. Write a balanced chemical equation for the neutralization of H 2 SO 4 (aq) with Cr(OH) 3 (aq). 9. Gastric juice, the digestive fluid produced in the stomach, contains hydrochloric acid...#Ca(OH)_2 + H_2SO_4 = H_2O + CaSO4#. The zero sum rule says that the addition of all charges in molecule is 0. For example. If you know the charge of Ca in CaSO4 you can determine the charge of SO4.H2SO4(aq) → H+ + HSO4^- HSO4^- <=> H+ + SO4^2- The presence of H+ keeps the weak HSO4^- from ionizing except to a very slight extent. Since the balanced equation shows that 3 moles of calcium chloride are involved in the reaction a total of 3 moles (3 x 1) of Ca2+ and 6 moles (3 x 2) of...HI(aq)+LiOH(aq)→LiI(aq)+H2O(l). what happens when H+ mix with OH 0.106 mol Ba(OH)2. A 33.00mL sample of an unknown H3PO4 solution is titrated with a 0.110M NaOH solution. weak acids. strong electrolytes example. NaCl (aq). Strong base, Ca(OH)2.Ca(OH)2 is the base for this reaction. Bases on the observations recorded in the table answer the following questions : (a) Which is the most reactive metal? (b) Which is the least reactive metal? (c) What would be observed if metal D was added to a solution of copper(II)sulphate? (d) What would be...
How do you balance and translate this reaction: Ca(OH)_2(aq)...
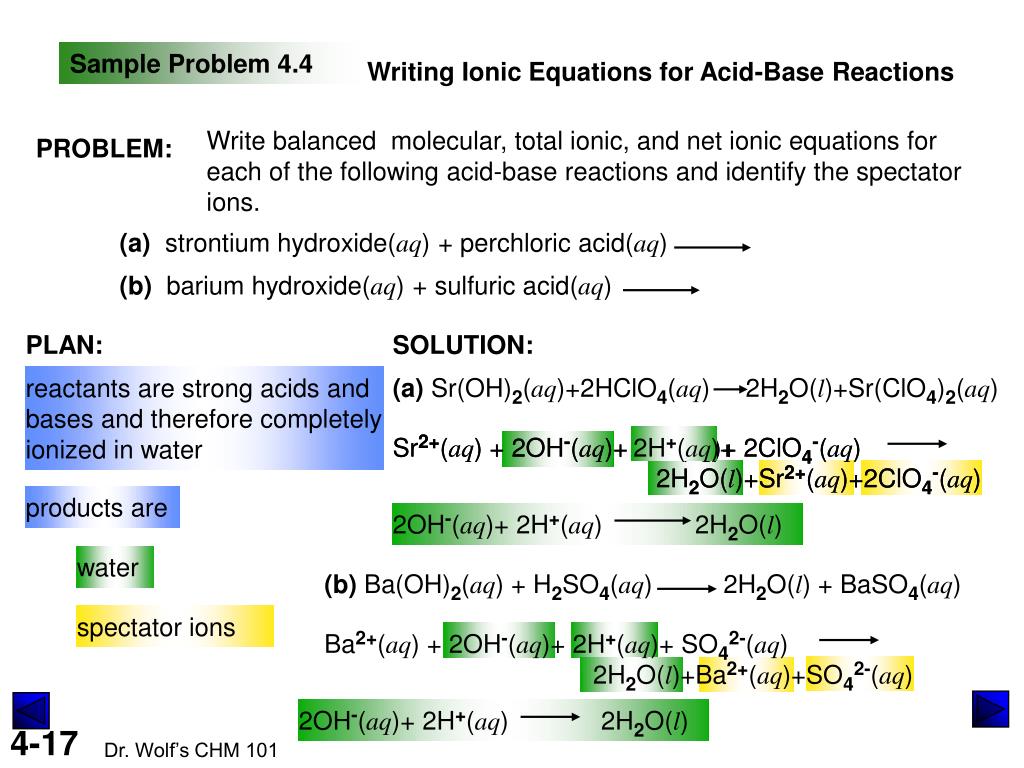
Identify all of the phases in your answer: Ca(OH)2( a q )+CH3CO2H( a q )â†' Express your answer... HNO3( a q )+Na2SO3( a q )â†' Express your answer as a chemical equation.Balance the reaction of Ca(OH)2 + H2SO4 = CaSO4 + H2O using this chemical equation balancer!In this video we'll balance the equation Ca(OH)2 + H2SO4 = CaSO4 + H2O and provide the correct coefficients for each compound.To balance Ca(OH)2 + H2SO4...This is a neutralization reaction so the resulting product will be water and salt. Reaction - Calcium Hydroxide + Sulfuric Acid → Calcium Sulfate + Water. Balanced - Ca(OH)2 + H2SO4 → CaSO4 + 2H2O.

What is the complete ionic equation for the reaction that occurs... - Quora
H₂SO₄(aq) + Ca(OH)₂(aq) ---> CaSO₄(aq) + 2H₂O(/) Which substance is the base in the reaction? prettygirlmicah prettygirlmicah. Ca(OH)2 (aq) because of elements oxygen and hydrogen.Ca(OH)2 - Calcium hydroxide. Other names: {{ubl , Slaked lime , Calcium hydrate. show more. Caustic lime , Hydrated lime , Ca(OH)2 , Hydralime , Calcium dihydroxide. show less. Appearance: White powder ; Colourless crystals or white powder ; White, odorless powder.H2SO4+Ca(OH)2=CaSO4+2H2O Полное: 2H⁺+SO4²⁻+Ca²⁻+2OH⁻=CaSO4+2H2O Краткого нет.2H + (aq) +2OH - (aq) Æ 2H 2 O(l) (because they are all the same, the coefficients may be left out) b must add up to zero, and there are two N atoms, so the two N atoms combined must add up to -4 Provided by Tutoring Services 12 Aqueous Reactions c. Ca(s) + F 2 (g) Æ CaF 2 (s) i. Reactant ox...This is a chemical reaction equationH2SO4(aq) + Ca(OH)2(s) --> CaSO4(s) + 2H2O(l). Because it will react violently in water producing hydrogen gas in a highly exothermic reaction which often causes ignition of the hydrogen gas liberated: 2Nas + 2H2Ol --> H2,g + 2(NaOH)aq The kerosene used for...
Yahoo Answers is shutting down on May 4th, 2021 (Eastern Time) and beginning April 20th, 2021 (Eastern Time) the Yahoo Answers website will be in read-only mode. There will be no changes to other Yahoo properties or services, or your Yahoo account. You can find more information about the Yahoo Answers shutdown and how to download your data on this help page.
Acids and Bases

Balanced Equation For Reaction Of Carbon Dioxide With ...

Chemistry Archive | July 19, 2017 | Chegg.com
PPT - Chapter 4 The Major Classes of Chemical Reactions ...
Solved: Sulfuric Acid Is A Strong Acid And Dissociates In ...

Group 2 Elements - Trends and Properties

Lengkapi reaksi reaksi berikut: a) ba(oh)2(aq)+hcl(aq) b ...

Solved: Based On The Solubility Rules Given In Class, Clas ...

AQA Chemistry C2 Revision

PPT - Neutralization Reactions PowerPoint Presentation ...

Answered: 1. Write balanced equations for the… | bartleby

Complete and balance each of the following equations for ...

Answered: NaOH(aq) + H2SO4(aq)= balanced eqaution… | bartleby

Solved: 23. Write A Complete Ionic And Net-ionic Equation ...

Solved: 5- Complete And Balance The Equation For Each Of T ...

Ôn tập Na2CO3 + H2SO4 - Công Ty TNHH Hóa Chất Hanimex

PPT - Theoretical Yield: Which Reactant is Limiting ...
⚗️Look at the reaction below. Which substance is the base ...

PPT - Single Displacement Reactions PowerPoint ...

Solved: Give The NET Ionic Equation For The Reaction (if A ...

setarakan persamaan reaksi berikut ini : a. NaOH(aq ...


Aucun commentaire:
Enregistrer un commentaire
Remarque : Seul un membre de ce blog est autorisé à enregistrer un commentaire.